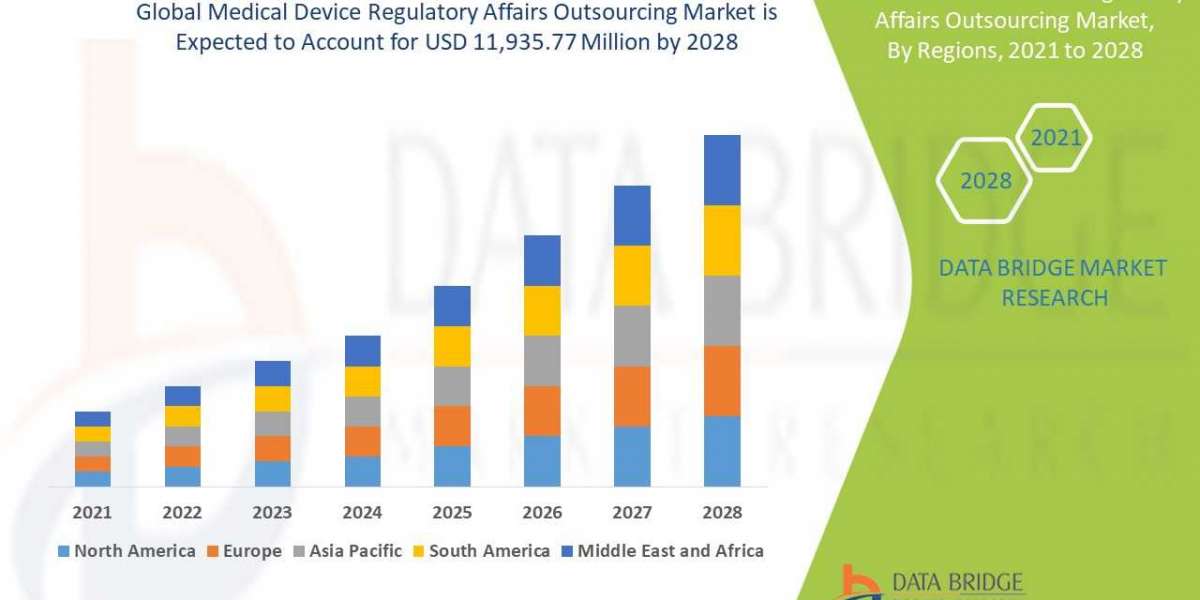
The global medical device regulatory affairs outsourcing market is expected to gain market growth in the forecast period of 2021 to 2028 with a CAGR of 12.8% in the forecast period of 2021 to 2028 and is expected to reach USD 11,935.77 million by 2028
Get a sample of the report
The top-notch global medical device regulatory affairs outsourcing market report endows with a profound overview of product specification, product type, production analysis, and technology by taking into consideration the major factors such as revenue, cost, and gross margin. This market report is the best source that gives CAGR values of 12.8% with variations during the forecast period of 2021-2028 for the market. The study encompasses market drivers and restraints by using SWOT analysis, along with their impact on the demand over the forecast period. Geographical areas such as North America, South America, Europe, Asia-Pacific, and Middle East Africa are also considered for the market analysis. The significant global medical device regulatory affairs outsourcing market has been prepared based on the market type, size of the organization, availability on-premises, and the end-users organization type.
A world-class global medical device regulatory affairs outsourcing market report contains basic, secondary, and advanced information related to the global status, recent trends, market size, sales volume, market share, growth, future trends analysis, segment, and forecasts from 2021 - 2028. Furthermore, this business report presents the data and information for actionable, most recent, and real-time market insights which make it easier to even take critical business decisions. Market research analysis data included in this industry report lend a hand to businesses for planning strategies related to investment, revenue generation, production, product launches, costing, inventory, purchasing, and marketing. Gglobal medical device regulatory affairs outsourcing market business report gives knowledge and insights about the new regulatory environment which suits the organizations
Market Analysis and Insights: Global Medical Device Regulatory Affairs Outsourcing Market
- The medical device regulatory affairs outsourcing market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses that the market is growing with a CAGR of 12.8% from 2021 to 2028 and is expected to reach USD 11,935.77 million by 2028.
- The strategic initiative for geographical expansions is anticipated to drive the growth of the medical device regulatory affairs outsourcing market.
- Outsourcing is an important part of every pharmaceutical and biotechnology companies’ value chain during research and development (RD).
- The regulatory affairs outsourcing services entail medical writing and publication of regulatory documentation by professional medical authors, quality control (QC) auditors and publishers who contribute to high-quality clinical research projects.
- The demand for regulatory services outsourcing has been fueled by a substantial increase in clinical studies conducted in emerging economies, providing a healthy platform for this industry's growth.
Medical Device Regulatory Affairs Outsourcing Market Scope and Market Size
On the basis of services, the medical device regulatory affairs outsourcing market is segmented into regulatory affairs services, quality consulting and medical writing. In 2021, the regulatory affairs services segment is expected to dominate the medical device regulatory affairs outsourcing market because of the increased adoption of regulatory affairs outsourcing by key medical device companies.
- On the basis of product, the medical device regulatory affairs outsourcing market is segmented into finished goods, electronics and raw material. In 2021, the finished goods segment is expected to dominate the medical device regulatory affairs outsourcing market due to the increased adoption of regulatory affairs outsourcing for the finished goods by major medical device companies.
- On the basis of device type, the medical device regulatory affairs outsourcing market is segmented into class I, class II and class III. In 2021, the class I segment is expected to dominate the medical device regulatory affairs outsourcing market because of the rising demand for medical devices worldwide to treat patients with chronic diseases.
- On the basis of application, the medical device regulatory affairs outsourcing market is segmented into cardiology, diagnostic imaging, orthopedic, IVD, ophthalmic, general and plastic surgery, drug delivery, dental, endoscopy, diabetes care and others. In 2021, the cardiology segment is expected to dominate the medical device regulatory affairs outsourcing market because of the increased adoption of regulatory affairs outsourcing for the class III medical devices by key medical device companies.
- On the basis of end user, the medical device regulatory affairs outsourcing market is segmented into small medical device company, medium medical device company and large medical device company. In 2021, the medium medical device company segment is expected to dominate the medical device regulatory affairs outsourcing market due to the rising demand for medical devices worldwide.
Global Medical Device Regulatory Affairs Outsourcing Market Country Level Analysis
Global medical device regulatory affairs outsourcing market is analyzed and market size information is provided by the country, services, product, device type, application and end user as referenced above.
The countries covered in the global medical device regulatory affairs outsourcing market report are the U.S., Canada, Mexico, Germany, U.K., France, Italy, Spain, Netherlands, Russia, Switzerland, Belgium, Turkey, Ireland, rest of Europe, Japan, China, Australia, India, South Korea, Singapore, Indonesia, Thailand, Malaysia, Philippines, rest of Asia-Pacific, Brazil, Argentina, rest of South America, Saudi Arabia, South Africa, UAE, Israel, Egypt and rest of Middle East and Africa.
Competitive Landscape and Medical Device Regulatory Affairs Outsourcing Market Share Analysis
The medical device regulatory affairs outsourcing market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breadth, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus related to the global medical device regulatory affairs outsourcing market.
The major companies covered in the global medical device regulatory affairs outsourcing market report are Parexel International Corporation, North American Science Associates, Inc., SGS SA, Pace Analytical Services, LLC, Trilogy Writing Consulting GmbH, Creganna (a subsidiary of TE Connectivity), American Preclinical Services, LLC, Intertek Group plc, WuXi AppTec, Charles River Laboratories, Celestica Inc., Freyr, Cactus Communications, Cekindo Business International, Eurofins Scientific, TÜV SÜD, Sterigenics U.S., LLC – A Sotera Health company, TE Connectivity, FLEX LTD., Heraeus Holding, Integer Holdings Corporation, Nortech Systems, Inc., IQVIA, Covance, Plexus Corp., Sanmina Corporation, OMICS International, Tecomet, Inc., East West Manufacturing, Jabil Inc., Omron Corporation among other global and domestic players. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
Get Full Access to the Report
MAJOR TOC OF THE REPORT
- Chapter One: Introduction
- Chapter Two: Market Segmentation
- Chapter Three: Market Overview
- Chapter Four: Executive Summary
- Chapter Five: Medical Device Regulatory Affairs Outsourcing Market
- Get TOC Details
Browse Related Reports@
https://www.databridgemarketresearch.com/reports/global-leprosy-market
https://www.databridgemarketresearch.com/reports/global-soft-tissue-sarcomas-treatment-market
https://www.databridgemarketresearch.com/reports/global-panhypopituitarism-x-linked-market
https://www.databridgemarketresearch.com/reports/global-passive-matrix-liquid-crystal-display-market
https://www.databridgemarketresearch.com/reports/global-variable-refrigerant-flow-vrf-market
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact:
Data Bridge Market Research
Tel: +1-888-387-2818
Email: [email protected]