Clinical research is a critical component of advancing medical knowledge and improving patient care. It involves rigorous scientific investigation to develop new treatments, therapies, and drugs. But at the heart of clinical research are the patients—the individuals who participate in trials to test these medical innovations. Patient advocacy groups play a significant role in supporting, educating, and empowering patients in the clinical research process. In this article, we'll explore the importance and impact of patient advocacy groups in clinical research.
Understanding Clinical Research
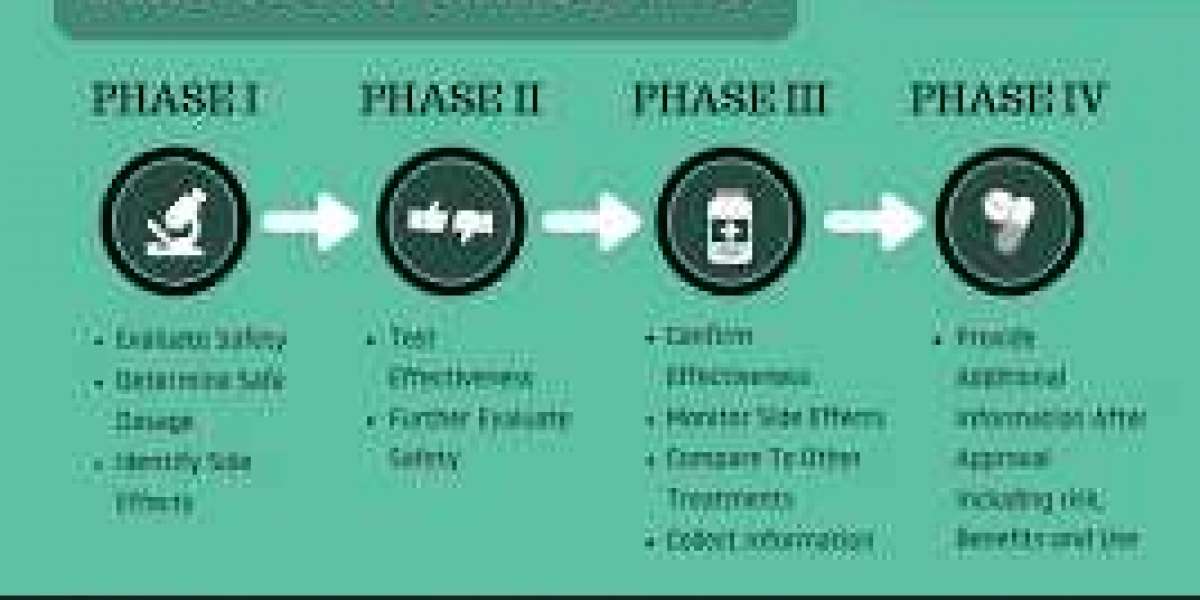
Clinical research is the systematic study of medical interventions on human participants. These interventions can range from new drug trials and medical devices to behavioral interventions and surgical procedures. Clinical trials are a common form of clinical research, where new treatments are tested for safety and efficacy.
The Role of Patients in Clinical Research
Patients are at the core of clinical research. They volunteer to participate in trials, often with the hope of improving their own health or the health of others with similar conditions. Their participation provides valuable data that informs medical decisions, regulatory approvals, and treatment guidelines.
Challenges Faced by Patients in Clinical Research
Participating in clinical trials can be a complex and challenging journey for patients. They may encounter various obstacles, including:
Lack of Information: Patients may not have access to clear and concise information about clinical trials, making it difficult to make informed decisions.
Logistical Challenges: Trials can involve extensive travel, time commitments, and expenses, which can be burdensome for participants and their families.
Emotional Impact: The uncertainty and potential risks associated with clinical trials can take an emotional toll on patients and their loved ones.
Lack of Support: Some patients may feel isolated during their participation in clinical trials, lacking a support system that understands their experience.
The Role of Patient Advocacy Groups
Patient advocacy groups, also known as patient advocacy organizations, are nonprofit organizations dedicated to supporting individuals living with specific medical conditions or facing particular health challenges. These groups play a crucial role in clinical research in several ways:
Education: Patient advocacy groups provide patients with information about clinical trials, including their rights, potential benefits, and risks. They bridge the gap between complex medical research jargon and patient-friendly language.
Empowerment: These groups empower patients to become active participants in their healthcare decisions. They encourage patients to ask questions, seek second opinions, and advocate for themselves.
Support: Patient advocacy groups offer emotional support and a sense of community. They connect patients with others who are going through similar experiences, reducing feelings of isolation.
Awareness: These groups raise awareness about specific medical conditions and the importance of clinical research. They promote the value of clinical trials in advancing medical knowledge.
Advocacy: Patient advocacy organizations advocate for policies and regulations that prioritize patient rights and access to clinical trials. They work to remove barriers that hinder patient participation.
Clinical Research Blogs: A Valuable Resource
Clinical research blogs serve as a valuable resource for patients seeking information about ongoing trials, the latest developments in medical research, and updates from patient advocacy groups. These blogs provide a platform for sharing stories of patient participation, success stories, and challenges faced.
The Role of Software Development
Software development plays a behind-the-scenes role in clinical research, facilitating data collection, analysis, and the management of trial information. Updates in software development are essential for streamlining processes and ensuring the secure handling of patient data.
Conclusion
Patient advocacy groups are instrumental in ensuring that patients are informed, supported, and empowered throughout their clinical research journey. Their dedication to improving the lives of individuals with specific medical conditions makes a significant impact on the success of clinical trials and the advancement of medical science. As clinical research continues to evolve, the collaboration between patients, advocacy groups, and the healthcare industry will remain a crucial factor in driving progress and improving patient care.