Being a proficient and all-inclusive Medical Device Testing Market report puts a light on primary and secondary drivers, market share, leading segments, possible sales volume, and geographical analysis. This market research report is a significant source of information about the industry, important facts and figures, expert opinions, and the newest developments across the globe. This report has reviews about key players in the market, major collaborations, merger and acquisitions along with trending innovation and business policies.
Furthermore, the world Medical Device Testing Market report deeply analyses the potential of the market with respect to current state of affairs and the future prospects by considering all aspects of Proprietary HMI (Human Machine Interface) Software Market industry. Not to mention, in this competitive market place, market research report has a very central role to play by offering important and consequential market insights for the business. The market drivers and restraints have been explained using SWOT analysis. With an absolute devotion and commitment, Europe Medical Device Testing market report has been provided with the best reasonable service and recommendations which can be relied upon confidently.
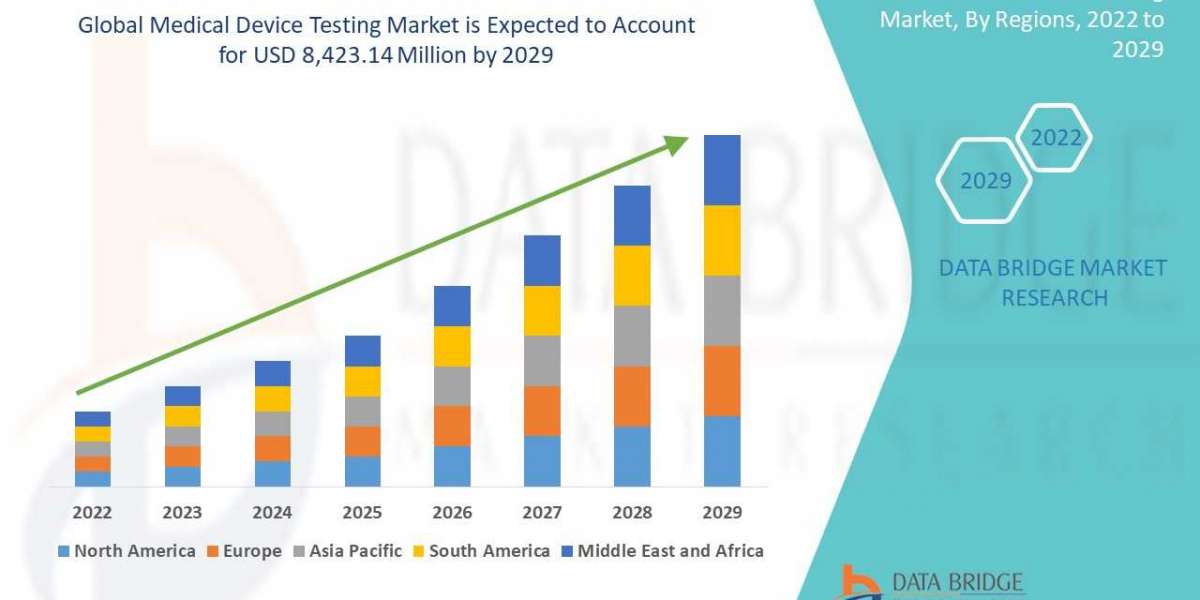
Global medical device testing market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 10.8% in the forecast period of 2022 to 2029 and is expected to reach USD 8,423.14 million by 2029 from USD 3,832.27 million in 2021.
Download Sample PDF Copy of this Report to understand structure of the complete report@ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-medical-device-testing-market
Market Overview:
Medical device testing is the process of demonstrating that the device is reliably and safely perform in use. In new product development, extensive design validation testing is applied. This includes performance testing, toxicity and chemical analysis, and sometimes human factors or even clinical testing. Ongoing quality assurance testing is generally more limited. This usually include dimensional checks, some functional tests, and packaging verification. Various types of medical testing services are available there in the market such as inspection services, certification services and among others.
Some of the major players operating in the medical device testing market are Intertek Group plc, SGS SA, Bureau Veritas, TUV SUD, TUV Rheinland, Pace, Charles River Laboratories, Biomedical Device Labs, UL LLC, North American Science Associates, LLC, Medistri SA, WuXi AppTec, NSF, Labcorp, Eurofins Scientific, Nelson Laboratories, LLC- A Sotera Health company, Gateway Analytical, ITC ZLIN, Element Materials Technology, EndoLab Mechanical Engineering GmbH, Hohenstein, Medical Engineering Technologies Ltd., Bioneeds, Cigniti, Arbro Pharmaceuticals Private Limited Auriga Research Private Limited, Q Laboratories, IMR Test Labs among others.
Global medical device testing market is segmented into service type, testing type, phase, sourcing type, device class and product. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Service Type
Testing services
Inspection services
Certification services
On the basis of service type, the global medical device testing market is segmented into testing services, inspection services and certification services.
Testing Type
Physical testing
Chemical/biological testing
Cybersecurity testing
Microbiology and sterility testing
Others
On the basis of testing type, the global medical device testing market is segmented into physical testing, chemical/biological testing, cybersecurity testing, microbiology and sterility testing and others.
Phase
Preclinical
Clinical
On the basis of phase, the global medical device testing market is segmented into preclinical and clinical.
Browse Full Report Along with Facts and Figures @ https://www.databridgemarketresearch.com/reports/global-medical-device-testing-market
TABLE OF CONTENTS
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Research Methodology
Part 04: Market Landscape
Part 05: Pipeline Analysis
Part 06: Market Sizing
Part 07: Five Forces Analysis
Part 08: Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers and Challenges
Part 13: Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Browse Trending Reports:
About Data Bridge Market Research:
An absolute way to predict what the future holds is to understand the current trend! Data Bridge Market Research presented itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are committed to uncovering the best market opportunities and nurturing effective information for your business to thrive in the marketplace. Data Bridge strives to provide appropriate solutions to complex business challenges and initiates an effortless decision-making process. Data Bridge is a set of pure wisdom and experience that was formulated and framed in 2015 in Pune.
Data Bridge Market Research has more than 500 analysts working in different industries. We have served more than 40% of the Fortune 500 companies globally and have a network of more than 5,000 clients worldwide. Data Bridge is an expert in creating satisfied customers who trust our services and trust our hard work with certainty. We are pleased with our glorious 99.9% customer satisfaction rating.
Contact Us: -
Data Bridge Market Research
US: +1 888 387 2818
United Kingdom: +44 208 089 1725
Hong Kong: +852 8192 7475
Email: – [email protected]